If you are looking for Airnova | GMP – Annex 1 you've came to the right place. We have 15 Pictures about Airnova | GMP – Annex 1 like Michael Herd on LinkedIn: Glove leak testing, what changes with the new, EU GMP-ANNEX1 and also The Long Awaited Revision 2 to EU Annex 1 | IVT. Here you go:
Airnova | GMP – Annex 1
 www.airnova.it
www.airnova.it gmp annex airnova normative sei
EU GMP Annex 1 Revision 2020, Manufacture Of Sterile Medicinal Products
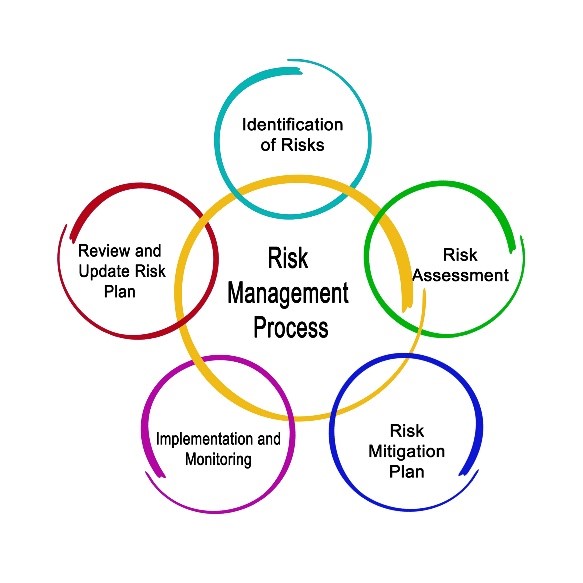 www.honeymangroup.com
www.honeymangroup.com annex contamination eu gmp strategy control risk qrm management revision quality manufacture sterile training implement significant facility changes document across
Annex 1 — Personnel Licensing Chapter 1
 www.servetbasol.com
www.servetbasol.com annex chapter
Annex 1
 www.imorules.com
www.imorules.com annex
EU GMP Annex 1 Revision 2020, Manufacture Of Sterile Medicinal Products
 www.honeymangroup.com
www.honeymangroup.com annex revision eu sterile gmp mitigate numerous references formation biofilm pharmacopeia within current there
Michael Herd On LinkedIn: Glove Leak Testing, What Changes With The New
 www.linkedin.com
www.linkedin.com The Long Awaited Revision 2 To EU Annex 1 | IVT
 www.ivtnetwork.com
www.ivtnetwork.com annex eu awaited revision long
EU GMP Annex 1 - Insights And Updates - Particle Measuring Systems
 www.pmeasuring.com
www.pmeasuring.com annex gmp
(PDF) EU GMP Annex 1 - The New Draft And Implications For Sterile
 www.researchgate.net
www.researchgate.net gmp annex implications sterile
Concerns Surrounding Annex 1 Proposed Revisions
 www.europeanpharmaceuticalreview.com
www.europeanpharmaceuticalreview.com annex gmps
Highlights From"Current Challenges In Aseptic Processing, Potential
sett aseptic pda allegati programma
How Ready Are Your Cleanrooms For Annex 1 Updates? | American
 www.americanpharmaceuticalreview.com
www.americanpharmaceuticalreview.com annex cleanrooms contamination microbial settle
Annex 1 Latest Draft Revision Updates - Particle Measuring Systems
 www.pmeasuring.com
www.pmeasuring.com annex revision viable requirements
GMP Annex 1 Revision 2022 - Buy Revision™ 20 On Official Website Today
 stopp-fruhstuck.com
stopp-fruhstuck.com annex gmp eu draft potential impact cleaning revisions
EU GMP-ANNEX1
 studylib.net
studylib.net Gmp annex implications sterile. Annex revision viable requirements. Eu gmp annex 1 revision 2020, manufacture of sterile medicinal products





No comments: